Netmeds First Membership
Quick Links
Introduction About EDASTAR INJECTION
EDASTAR INJECTION contains Edaravone which belongs to group of medicines called Antioxidants. EDASTAR INJECTION is used to manage amyotrophic lateral sclerosis (nervous system disease that weakens muscles and impacts physical function) in affected individuals.
EDASTAR INJECTION is not recommended for use if you are allergic to Edaravone.
Before receiving EDASTAR INJECTION, inform your doctor if you have lung problems (such as asthma), kidney problems (such as impaired renal insufficiency), and/or liver problems (such as mild/moderate/severe hepatic insufficiency).
EDASTAR INJECTION should be used with caution in pregnant and breastfeeding women only if it is clearly necessary since there is no adequate data to determine safety and efficacy of the medicine.
EDASTAR INJECTION is not recommended for use in children and adolescents (below 18 years) as there is no adequate data to determine the safety and efficacy of the medicine in children. It is advised to be used with caution in elderly patients (aged 65 years/above) after consulting the doctor.
The most common side effects of receiving EDASTAR INJECTION are confusion, gait disturbance, headache, dermatitis, eczema, and/or tinea infection (fungal infection of skin). Consult and inform your doctor if any of these symptoms worsen.
Uses Of EDASTAR INJECTION
- Manage amyotrophic lateral sclerosis in affected individuals
How EDASTAR INJECTION Works
EDASTAR INJECTION works by relieving the effects of oxidative stress (condition that occurs due to imbalance of free radicals and antioxidants in the body during ageing process) which results in delayed nerve damage associated with the symptoms of amyotrophic lateral sclerosis condition thus preserving the motor function and managing the associated symptoms of the condition in affected individuals.
How to use EDASTAR INJECTION
EDASTAR INJECTION will be given to you only by a doctor or a nurse into a vein as an intravenous infusion. Your doctor will decide the correct dose and duration for you depending upon your age, body weight and disease condition.
Side Effects Of EDASTAR INJECTION
Common
- confusion
- gait disturbance
- headache
- dermatitis
- eczema
- signs of glycosuria (condition marked by excess sugar in urine) such as extreme hunger, accidental urination, nighttime urination
- tinea infection (fungal infection of skin)
Rare
Stop receiving EDASTAR INJECTION and contact your doctor immediately if you have:
- signs of hypersensitivity reactions (immune mediated response to an allergen) such as red itchy welts, breathing problems, itching, swelling of lips/tongue/face, dizziness, wheezing, and/or fainting
- signs of sulfite allergic reactions such as asthma attack (in people with a history of asthma), red itchy welts, breathing problems, itching, swelling of lips/tongue/face, dizziness, wheezing, and/or fainting
How To Manage Side Effects
Headache:
If EDASTAR INJECTION causes headache, then take rest and drink plenty of fluids. Try to avoid drinking alcohol. Ask your doctor to recommend a painkiller. Headaches should usually go away after the first week of taking EDASTAR INJECTION. If it lasts more than a week or severe, inform your doctor.
Warning & Precautions
Pregnancy
EDASTAR INJECTION should be used with caution during pregnancy and in women planning for pregnancy only if it clearly necessary since there is no adequate data to determine safety and efficacy of the medicine. Consult your doctor before receiving the medicine.
Breastfeeding
EDASTAR INJECTION should be used with caution in breastfeeding women only if it is clearly necessary as there is no data to determine its safety and efficacy. Consult your doctor before receiving the medicine.
Kidney
EDASTAR INJECTION should be used with caution in patients with kidney problems such as renal insufficiency. Consult your doctor before receiving the medicine.
Liver
EDASTAR INJECTION should be used with caution in patients with liver problems such as mild/moderate/severe hepatic insufficiency. Consult your doctor before receiving the medicine.
Allergy
Do not receive EDASTAR INJECTION if you are allergic to edaravone, and/or any other ingredients of this medicine. It should be used with caution in patients allergic to sulfites. Consult and inform your doctor before receiving the medicine.
Lungs
EDASTAR INJECTION should be used with caution in patients with lung problems such as asthma. Consult your doctor before receiving the medicine.
Others
Use in Pediatrics:
EDASTAR INJECTION is not recommended for use in children and adolescents (below 18 years) as there is no adequate data to determine the safety and efficacy of the medicine in children. Consult your doctor before administering the medicine to the child.
Use in Geriatrics:
EDASTAR INJECTION should be received with caution in elderly patients (aged above 65 years). Consult and inform your doctor before receiving the medicine.
Interactions
A. Drug-Drug interactions:
Before taking EDASTAR INJECTION, inform your doctor if you are taking:
- CYP enzyme inhibitors (medicines that inhibit cyp enzyme which is responsible for cholesterol & steroid production) Ex. clarithromycin, diltiazem, erythromycin, itraconazole
- UGT inhibitors (medicines that inhibit metabolism of drugs) Ex. diclofenac, phenylbutazone
Over dosage:
EDASTAR INJECTION will be administered to you only by a doctor or a nurse in a hospital, and so it is unlikely to receive an overdose. However, consult your doctor or nurse if you experience any unusual symptoms.
Synopsis
| Drug | : | Edaravone |
| Pharmacological Category | : | Antioxidants |
| Therapeutic Indication | : | Amyotrophic lateral sclerosis |
| Dosage Forms | : | Injection |
More Information
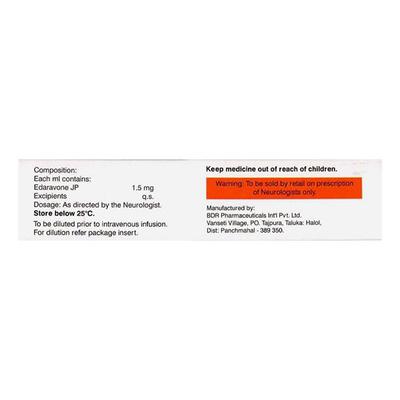
- Keep EDASTAR INJECTION out of reach of children
- Store EDASTAR INJECTION below 25°C
FAQs About EDASTAR INJECTION
What are the uses of EDASTAR INJECTION?
EDASTAR INJECTION is used to manage amyotrophic lateral sclerosis in affected individuals which is a progressive neuro muscular disease that weakens muscles and impacts physical function, generally characterized by symptoms such as difficulty while walking, tripping & falling, weakness in legs/feet/ankles, slurred speech, and/or trouble swallowing.
What are the side effects of EDASTAR INJECTION?
The most common side effects of receiving EDASTAR INJECTION are confusion, gait disturbance, headache, dermatitis, eczema, and/or tinea infection (fungal infection of skin). Contact your doctor if any of these symptoms worsen.
Is EDASTAR INJECTION safe to be used in pregnant and breastfeeding women?
Yes, EDASTAR INJECTION can be used but with caution during pregnancy only if it is clearly necessary. It is also advised to be used with caution in breast feeding only if needed since it is unknown whether the medicine may get excreted through breastfed milk. Consult your doctor before receiving the medicine.
How EDASTAR INJECTION works?
EDASTAR INJECTION works by relieving the effects of oxidative stress (condition that occurs due to imbalance of free radicals and antioxidants in the body during ageing process) which results in delayed nerve damage associated with the symptoms of amyotrophic lateral sclerosis condition thus preserving the motor function and managing the associated symptoms of the condition in affected individuals.
How much dose can EDASTAR INJECTION be received in a day?
EDASTAR INJECTION is generally given to you only by a doctor or a nurse into a vein as an intravenous infusion. Your doctor will decide the correct dose and duration depending on your age, body weight and disease condition.
References
1. Patrick A. Lewis, Jennifer E. Spillane. The motor neuron diseases and amyotrophic lateral sclerosis. The Molecular and Clinical Pathology of Neurodegenerative Disease. Edition 2018. Page: 178.
2. R Bhandari, A Kuhad. Edaravone: a new hope for deadly amyotrophic lateral sclerosis. NIH National Library of medicine. National Center for Biotechnology Information. PubMed.gov. [Revised in June 2018] [Accessed on 19th May 2022] https://pubmed.ncbi.nlm.nih.gov/29998226/
3. Martin Paspe Cruz. Edaravone (Radicava). NIH National Library of medicine. National Center for Biotechnology Information. PMC PubMed Central. January 2018. [Accessed on 19th May 2022] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5737249/
4. Mitsubishi Tanabe Pharma Corporation. Drug and Health Product Register (HRES). [Revised in October 2018] [Accessed on 19th May 2022] https://pdf.hres.ca/dpd_pm/00047612.PDF
5. Taj Pharma. Edaravone Injection. [Accessed on 19th May 2022] https://tajpharma.in/portfolio-item/edaravone-injection-1-5mg/
Useful Diagnostic Tests
- Blood test
- Magnetic Resonance Imaging (MRI)