Netmeds First Membership
Introduction About TYVALZI 30MCG INJECTION
TYVALZI Injection is used in the management of Acute Cerebral Ischemic Stroke, a condition in which the loss of blood supply to the brain manages brain tissue from receiving oxygen and nutrients, resulting in potential brain damage. It contains a medicine called Sovateltide which stimulates the production of neural progenitor cells in the brain, supporting the generation of new brain cells and blood vessels post-cerebral ischemic stroke. This process enhances blood flow to the brain, promoting the survival of brain cells.
Studies have shown that, TYVALZI has anti-inflammatory, and antioxidant activity which helps in limiting brain cell damage and potentially restoring neurological and motor function in patients following a stroke.
TYVALZI Injection will be given to you only a trained doctor or by any competent healthcare professional into your veins (i.v.) as an intravenous bolus over one minute.
While undergoing therapy with TYVALZI Injection, it is important to take all doses exactly in the same dosing intervals as suggested by your doctor to see better results. Before taking this medicine, inform your doctor if you any bleeding in the brain or any other pre-existing disease conditions as a precaution.
There is no study information regarding the use of TYVALZI during pregnant, breastfeeding and in children (aged below 18 years). Therefore, inform your doctor before this medicine is administered to you.
TYVALZI Injection is generally well tolerated. If you experience any unpleasant side effects, consult your doctor.
Uses Of TYVALZI 30MCG INJECTION
- Management of Acute Cerebral Ischemic Stroke
How TYVALZI 30MCG INJECTION Works
TYVALZI Injection contains a medicine called Sovateltide which is a neural progenitor cell therapeutic agent. It works by making your body produce neural progenitor cells - a type of cells in the brain that has the potential to develop into essential brain cells (various types of glial and neuronal cells) that populate the CNS.
This action of Sovateltide help in production of new brain cells and blood vessels, following cerebral ischemic stroke, hence increasing blood flow to the brain and maintains brain cells survival.
How to use TYVALZI 30MCG INJECTION
TYVALZI Injection should be used under medical supervision.
It will be given to you only a trained doctor or healthcare professional into your veins (i.v.) as an intravenous bolus over one minute.
Therapy with TYVALZI should be initiated within 24 hours of stroke onset, after the first day of management, repeat doses should be administered on day 3 and day 6 of the stroke. A total of nine doses of Tyvalzi should be administered. Therefore, it is important to closely follow the dosing information advised by your doctor for maximum benefits.
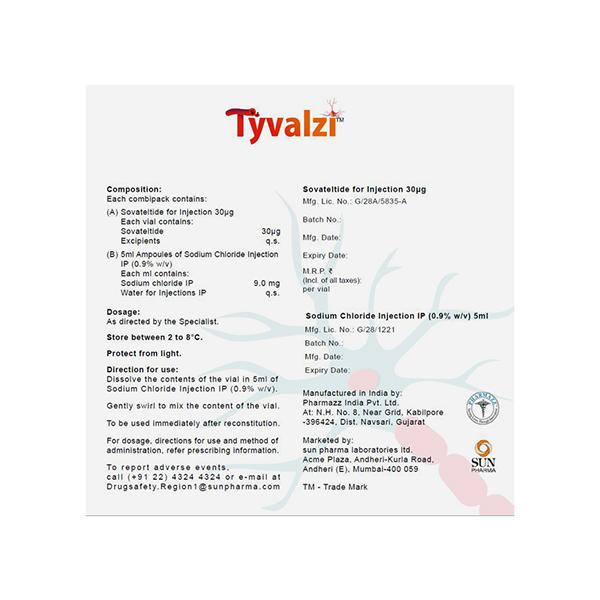
The contents of TYVALZI Injection should be reconstituted in 5ml of normal saline (sodium chloride injection). Gently swirl to mix the contents of the vial.
Side Effects Of TYVALZI 30MCG INJECTION
Current studies have shown that, TYVALZI Injection is well tolerated with minimal side effects as mentioned below. Consult your doctor if they trouble you.
How To Manage Side Effects
Nausea And Vomiting
Managing vomiting at home involves primarily focusing on hydration and comfort. Start by sipping clear fluids like water, electrolyte solutions, or ginger tea to manage dehydration and ease nausea. Rest in a comfortable position with your head elevated to alleviate discomfort. Avoid triggers like strong smells or heavy foods. Over-the-counter medications may help relieve symptoms, but consult a healthcare professional first, especially if you have underlying health conditions. Monitor symptoms closely and seek medical attention if vomiting persists, is severe, or is accompanied by other concerning symptoms like severe abdominal pain or signs of dehydration.
Warning & Precautions
Pregnancy
Consult your doctorThere are no well-controlled studies regarding the use of TYVALZI Injection (Sovateltide) in pregnant women. Therefore, before your doctor could suggest therapy with this medicine, inform your doctor if you are pregnant or think you may be pregnant as a precaution.
Breastfeeding
Consult your doctorThere are no well-controlled studies regarding the use of TYVALZI Injection (Sovateltide) in breastfeeding women. Therefore, before your doctor could suggest therapy with this medicine, inform your doctor if you are nursing.
Driving and Using Machines
Use with CautionThere is no evidence from the clinical studies indicating that TYVALZI Injection (Sovateltide) affects the ability to drive and use machines
Allergy
ContraindicatedTYVALZI Injection (Sovateltide) should not be used in patients who are allergic to Sovateltide or to any of its excipients.
Use In Pediatrics
Consult your doctorThe safety and effectiveness of TYVALZI Injection have not been established in children and adolescents (aged below 18 years). Your doctor will decide based on your child’s health condition.
Use In Geriatrics
Use with CautionTYVALZI Injection should be used with caution in elderly patients (aged 70 years and above). Therefore, consult your doctor for advice.
Others
TYVALZI Injection should be used with caution in patients with bleeding conditions of the brain such as:
- Intracranial hemorrhage (bleeding within the skull)
- Intracerebral hematoma (bleeding into the brain tissues)
- Intraventricular hemorrhage (bleeding into fluid areas or ventricles surrounded by the brain)
- Subarachnoid hemorrhage (Bleeding in the space between the brain and the membranes covering the brain)
- Epidural hemorrhage (bleeding between the inside of the skull and the dura matter (outer covering of the brain))
- Acute or chronic subdural hematoma (blood clogged between brain and outer covering)
Interactions
A. Drug-Drug Interactions:
Inform your doctor about all the medicines that you currently take as a precaution before TYVALZI is administered to you.
Overdosage:
TYVALZI Injection will be administered to you only by a doctor or a nurse in a hospital setting. Therefore, it is unlikely to receive an overdosage. However, if you experience any unpleasant symptoms, inform your doctor immediately.
More Information
Storage
- Keep TYVALZI Injection out of reach of children
- Store between 2-8?
- Protect from light
FAQs About TYVALZI 30MCG INJECTION
Q: What is the use of TYVALZI Injection?
A: TYVALZI Injection is used in the management of Acute Cerebral Ischemic Stroke. It works by supporting the generation of new brain cells and blood vessels post-cerebral ischemic stroke which enhances blood flow to the brain, promoting the survival of brain cells.
Q: What is the cost of Sovateltide in India?
A: Sovateltide (TYVALZI) costs Rs. 1300/- in India. The Price may vary from season to season based on the availability and current regulations.
Q: What is the use of Sovateltide injection?
A: Sovateltide is used in the management of Acute Cerebral Ischemic Stroke. It works by supporting the generation of new brain cells and blood vessels post-cerebral ischemic stroke which enhances blood flow to the brain, promoting the survival of brain cells.
Q: How to give TYVALZI Injection?
A: TYVALZI Injection will be given to you only a trained doctor or healthcare professional into your veins (i.v.) as an intravenous bolus over one minute. Therapy with TYVALZI should be initiated within 24 hours of stroke onset, after the first day of management, repeat doses should be administered on day 3 and day 6 of the stroke. A total of nine doses of Tyvalzi should be administered. Therefore, it is important to closely follow the dosing information advised by your doctor for maximum benefits.
Q: Can TYVALZI Injection be used in children?
A: The safety and effectiveness of TYVALZI Injection have not been established in children and adolescents (aged below 18 years). Your doctor will decide based on your child’s health condition.
Q: Is TYVALZI Injection safe?
A: TYVALZI Injection is well tolerated with minimal side effects uneasiness, sweating, abdominal discomfort and vomiting. Consult your doctor if they trouble you.
Q: How long does it take for TYVALZI Injection to show results?
A: Therapy with TYVALZI should be initiated within 24 hours of stroke onset, after the first day of management, repeat doses should be administered on day 3 and day 6 of the stroke. A total of nine doses of Tyvalzi should be administered. You might see an improvement in your condition after the completion of dosing schedule recommended by your doctor. However, the chances of complete recovery and recovery period may vary from person to person, based on the severity of brain cell damage.
Q: How to buy TYVALZI Injection online?
A: To buy TYVALZI Injection online from Netmeds, visit the Netmeds website or open the Netmeds mobile app. Look for " TYVALZI Injection" or enter the specific details of the medication you need. Add the medication to your cart. Proceed to checkout and follow the prompts to enter your shipping and payment details. It's important to ensure that you have a valid prescription for TYVALZI Injection before placing your order. Additionally, check the availability of the medication in your location and review Netmeds' shipping policies for any specific guidelines or restrictions.
Q: How TYVALZI Injection works?
A: TYVALZI Injection works by making your brain produce certain cells called neural progenitor cells, which has the capacity to develop into essential brain cells like various types of glial and neuronal cells and populate the central nervous system. This action, help in production of new brain cells and blood vessels, following cerebral ischemic stroke, hence increasing blood flow to the brain, promoting the survival of brain cells.
Q: Can TYVALZI Injection be used during pregnancy?
A: There are no well-controlled studies regarding the use of TYVALZI Injection (Sovateltide) in pregnant women. Therefore, before your doctor could suggest therapy with this medicine, inform your doctor if you are pregnant or think you may be pregnant as a precaution.
References
- Textbook of Pathology by Harsh Mohan. 6th Edition. 2010. Chapter 30. The Nervous System. Ischemic Brain Damage. Page – 879-881.
- Government of India. Directorate General of Health Services. Central Drugs Standard Control Organisation (CDSCO). FDA Bhawan, New Delhi. Permission to Conduct A prospective, multicentric, randomised, double-blinded, parallel, phase IV study to access safety and Efficacy of Tycamzzi TM (Sovateltide) in patients with acute ischemic stroke. 31st May 2023. [Accessed on 8th May 2024]. https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadCTApprovals/Pharmazz%20Solvateltide%20CT-06.pdf
- Sun Pharma and Pharmazz Inc. enter into licensing agreement for introducing Tyvalzi™ (Sovateltide) in India. [Accessed on 8th May 2024]. https://sunpharma.com/wp-content/uploads/2023/09/Press-Release-Sun-Pharma-and-Pharmazz-Inc.-licensing-agreement-for-Sovateltide-in-India.pdf
- Safety and Efficacy of Sovateltide (IRL-1620) in a Multicenter Randomized Controlled Clinical Trial in Patients with Acute Cerebral Ischemic Stroke. NIH. National Library of Medicine. National Center for Biotechnology Information. PMC. PubMed Central. January 2021. [Accessed on 8th May 2024]. https://pubmed.ncbi.nlm.nih.gov/33428177/
- Pharmazz Lnc. Sovateltide. A Novel First-in Class Drug to Treat Acute Cerebral Ischemic Stroke (ACIS). 2021. [Accessed on 8th May 2024]. https://www.pharmazz.com/cerebral-ischemic-stroke.php