Netmeds First Membership
Quick Links
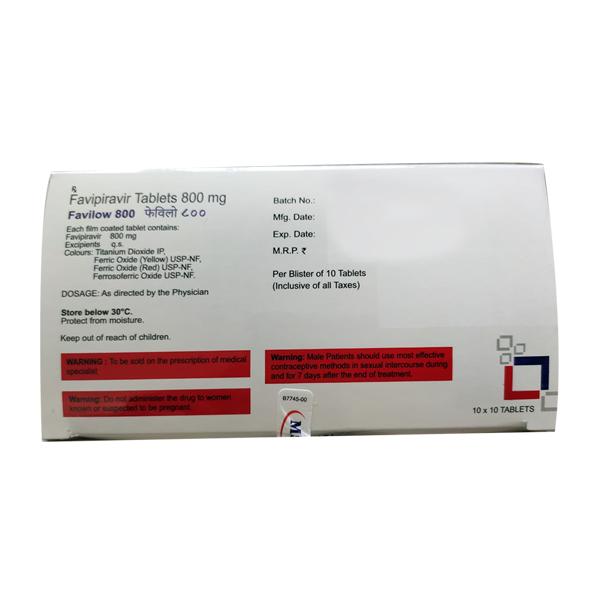
Introduction About FAVILOW 800 TABLET
FAVILOW 800 TABLET contains Favipiravir which belongs to the group of medicines called Antiviral drugs. It is used to manage patients with mild to moderate COVID-19 disease, which is an infectious disease caused by the SARS-CoV-2 virus that causes short-term, mild upper respiratory infection (common cold) associated with symptoms such as nasal obstruction, sneezing, runny nose and sometimes cough.
Prior to management with FAVILOW 800 TABLET, your doctor may want you to take several tests to understand your existing condition.
Before taking FAVILOW 800 TABLET, inform your doctor if you have gout. FAVILOW 800 TABLET is not recommended for use if you are allergic to Favipiravir or have severe liver or kidney impairment.
FAVILOW 800 TABLET is not recommended for use in pregnant and breastfeeding women, also it is not recommended for use in children and adolescents (aged below 18 years). It should be used with caution in elderly patients (aged 65 years or above).
The most common side effects of taking FAVILOW 800 TABLET is diarrhoea. Consult your doctor if your symptom worsens.
Uses Of FAVILOW 800 TABLET
- Manage mild to moderate COVID-19 disease
How FAVILOW 800 TABLET Works
FAVILOW 800 TABLET works by blocking the SARS-CoV-2 virus from multiplying which results in decreased viral load in the body thereby restricting the spread of infection and enhancing the recovery process from short-term, mild upper respiratory infections.
How to use FAVILOW 800 TABLET
Take FAVILOW 800 TABLET as advised by your physician. Swallow the medicine with a glass of water. Do not crush or chew the medicine. Your doctor will decide the correct dose and duration for you depending upon your age, body weight and disease condition.
Side Effects Of FAVILOW 800 TABLET
Common
- diarrhoea
Uncommon
- signs of allergic skin reactions such as rash
- nausea
- vomiting
- abdominal discomfort and pain
- eczema (skin inflammation)
- pruritus (itchy skin)
- duodenal ulcer
- bloody stool
- signs of gastritis (inflammation of stomach) such as burning ache in stomach
- asthma
- oropharyngeal pain such as pain or a scratchy sensation in the throat, difficulty while swallowing
- stuffy nose
- common cold
- tonsillar polyp (benign lesion of tonsil)
- pigmentation of skin (darkened patches or dark spots in skin)
- altered taste sensation
- bruise
- blurred vision
- eye pain
- vertigo (sudden internal or external spinning sensation)
- supraventricular extrasystole (additional heartbeats)
How To Manage Side Effects
Diarrhoea:
Drink lot of fluids such as water or fruit juice to keep yourself hydrated. Do not take any medicine on your own to manage diarrhea without consulting your doctor.
Nausea and vomiting:
Try to take FAVILOW 800 TABLET with or just after a meal or a snack. Stick to simple meals. Avoid eating oil rich or spicy foods. Consult your doctor if the symptom gets worse.
Stomach pain:
Try to take rest and relax. Eat and drink slowly or try to have smaller and frequent meals. Keep a heat pad on your stomach. If the symptom does not improve, consult your doctor.
Warning & Precautions
Pregnancy
FAVILOW 800 TABLET is not recommended for use in pregnant women. Consult your doctor before taking FAVILOW 800 TABLET.
Breastfeeding
FAVILOW 800 TABLET is not recommended for use in breastfeeding women. Consult your doctor before taking FAVILOW 800 TABLET.
Driving and Using Machines
Do not drive or operate any machines if your ability to concentrate or reaction is impaired due to side effects such as abnormal behavior or psychiatric symptoms after taking FAVILOW 800 TABLET.
Kidney
FAVILOW 800 TABLET is not recommended for use in patients with severe kidney impairment (kidney failure). Consult your doctor before taking FAVILOW 800 TABLET.
Liver
FAVILOW 800 TABLET is not recommended for use in patients with severe liver impairment (liver failure). Consult your doctor before taking FAVILOW 800 TABLET.
Allergy
Do not take FAVILOW 800 TABLET if you are allergic to Favipiravir or any other ingredients of this medicine.
Others
Before taking FAVILOW 800 TABLET, inform your doctor if you have:
- gout (complex form of joint pain)
- any other medical condition
Use in pediatrics:
FAVILOW 800 TABLET is generally not recommended for use in children and adolescents (aged below 18 years). Consult your child’s doctor for advice.
Use in geriatrics:
FAVILOW 800 TABLET should be used with caution in elderly patients (aged 65 years or above). Consult your doctor before taking FAVILOW 800 TABLET.
Interactions
A. Drug-Drug interactions:
Before taking FAVILOW 800 TABLET, inform your doctor if you are taking any of the following medicines:
- pyrazinamide (used to manage tuberculosis)
- famciclovir (antiviral medicine used to manage shingles)
- sulindac (medicine used to manage mild to moderate pain and help relieve symptoms of joint pain)
- theophylline (used to manage chronic pulmonary obstructive disease)
- oseltamivir (used to manage flu)
- raloxifene (used to manage osteoporosis)
- hydralazine (used in high blood pressure)
- acetaminophen (used to relieve pain and inflammation)
- medicines used to manage pregnancy (Ex. norethindrone, ethinyl estradiol)
- repaglinide (used to manage high blood sugar)
Overdosage:
If you or anyone else accidentally take too much of FAVILOW 800 TABLET, consult your doctor immediately or visit the nearby hospital.
Synopsis
| Drug | : | Favipiravir |
| Pharmacological Category | : | Modified pyrazine analog |
| Therapeutic Indication | : | Covid 19 disease |
| Dosage Forms | : | Tablet |
More Information
- Keep FAVILOW 800 TABLET out of reach of children
- Store below 30°C
FAQs About FAVILOW 800 TABLET
Is FAVILOW 800 TABLET safe to be used in children?
No, FAVILOW 800 TABLET is not recommended for use in children and adolescents. If FAVILOW 800 TABLET is accidentally ingested by a child or adolescent, take the child to hospital immediately and consult the doctor for further management.
Does FAVILOW 800 TABLET affect sperms?
FAVILOW 800 TABLET passes into the semen. Men must wear a condom during sexual intercourse during management with this medicine and up to 7 days after the completion of management. Ask your doctor if you have any more doubts.
Can FAVILOW 800 TABLET cause diarrhea?
Yes, if you experience diarrhea while taking FAVILOW 800 TABLET, drink lot of fluids such as water or fruit juice to keep yourself hydrated. Do not take any medicine on your own to manage diarrhea without consulting your doctor. Tell your doctor if you have continuous diarrhoea.
Can I get pregnant during management with FAVILOW 800 TABLET?
FAVILOW 800 TABLET is not recommended for use during pregnancy, so avoid planning for pregnancy during management with this medicine. Ensure implementation of extremely effective contraceptive methods during management and for 7 days after the completion of management. Discuss contraceptive methods with your doctor.
What precautions do I need to follow while taking FAVILOW 800 TABLET?
Patients taking FAVILOW 800 TABLET must inform their doctor about their pre-existing disease conditions like kidney, liver or heart problems. Patients should also inform if they are pregnant, breast-feeding, having any allergies or gout. Consult your doctor before taking FAVILOW 800 TABLET.
References
1. Shailendra K. Saxena. Current insight into the Novel Coronavirus Disease 2019 (COVID-19). Coronavirus Disease 2019 (COVID-19). Edition 2020. Page – 1, 2, 4, 5 & 112. https://www.google.co.in/books/edition/Coronavirus_Disease_2019_COVID_19/qovgDwAAQBAJ?hl=en&gbpv=0
2. Yousuke Furuta, Takashi Komeno, and Takaaki Nakamura. Favipiravir (T-705), a broad-spectrum inhibitor of viral RNA polymerase. NIH National Library of Medicine, National center for biotechnology information. PMC Pubmed Central. August 2017 [Accessed on 9th June 2022] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5713175/
3. Henrietta Papp, Zsofia Lanszki, Gyorgy M. Keseru, and Ferenc Jakab. Favipiravir for the treatment of COVID-19 in elderly patients—what do we know after 2 years of COVID-19. NIH National Library of Medicine, National center for biotechnology information. PMC Pubmed Central. May 2022 [Accessed on 9th June 2022] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9091543/
4. Cipla Ltd. Ciplenza Tablets. Favipiravir. Ciplamed [Revised in November 2020] [Accessed on 9th June 2022] https://www.ciplamed.com/content/ciplenza-tablets